Description
Abbott Nasal Self test 25 sterile nasal swabs a box
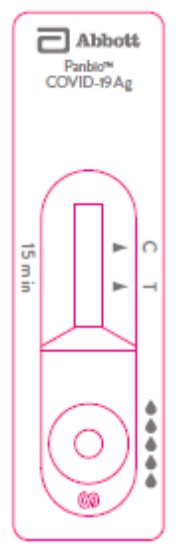
Panbio™ COVID-19 Ag Home Test Device
41FK11/41FK21 In vitro diagnostic rapid test for qualitative detection of SARS-CoV-2 antigen (Ag) (NASAL)
Compared to BinaxNow this Panbio Abbott test works with a plastic cassette and not with a paper enveloppe.
Expiry date:
Current Lot number expires on the 11th of December 2023
Introduction
The Omicron variant is a β-coronavirus, which is an enveloped non-segmented positive-sense RNA virus2
. It is spread by human-to-human transmission via droplets or direct contact. The main IVD assays used for COVID-19 employ real-time reverse transcriptase-polymerase chain reaction (RT-PCR) that takes a few hours.
. The availability of a cost-effective, rapid pointof-care diagnostic test is critical to enable healthcare professionals to aid in the
diagnosis of patients and prevent further spread of the virus BinaxNow and Panbio Antigen tests will play
a critical role in the fight against Omicron Advised use of Abbott by the WHO.
Test Principle
Unicef advises to use the Panbio™ COVID-19 Ag Rapid Test Device which contains a membrane strip, which
is pre-coated with immobilized anti-SARS-CoV-2 antibody on the test line and
mouse monoclonal anti-chicken IgY on the control line. Two types of conjugates
(human IgG specific to SARS-CoV-2 Ag gold conjugate (binds to the nucleocapsid
protein) and chicken IgY gold conjugate) move upward on the membrane
chromatographically and react with anti-SARS-CoV-2 antibody and pre-coated
mouse monoclonal anti-chicken IgY respectively. For a positive result, human IgG
specific to SARS-CoV-2 Ag gold conjugate and anti-SARS-CoV-2 antibody will
form a test line in the result window. Neither the test line nor the control line are
visible in the result window prior to applying the patient specimen. A visible control
line is required to indicate a test result is valid.
Intended Use
Panbio™ COVID-19 Ag Rapid Test Device is the preferred test in Qebec an in vitro diagnostic rapid test for
the qualitative detection of SARS-CoV-2 antigen (Ag) in human nasal swab
specimens from individuals who meet COVID-19 clinical and / or epidemiological
criteria. Canada Panbio protocol COVID-19 Ag Rapid Test Device is for professional use only
and is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection.
The product may be used in any laboratory and non-laboratory environment that
meets the requirements specified in the Instructions for Use and local regulation.
The test provides preliminary test results. Negative results don’t preclude SARSCoV-2 infection and they cannot be used as the sole basis for treatment or other management decisions. Negative results must be combined with clinical
observations, patient history, and epidemiological information. The test is not intended to be used as a donor screening test for SARS-CoV-2.
Kit Variants
• 41FK11 No 2D barcode printed on the contained test devices
• 41FK11 FIND Evaluation of the test device, which encodes traceability information for the product
• 195-000 BinaxNOW Ag CARD Kit (40 CT)
• 195-080 BinaxNOW Ag CARD CONTROL Kit (10 POSITIVE)
Materials Provided
• 25 Test devices with desiccant in individual foil pouch
• Buffer (1 x 9 ml/bottle)
• 25 Extraction tubes
• 25 Extraction tube caps
• 1 Positive control swab
• 1 Negative control swab
• 25 Sterilized nasal swabs for sample collection
• 1 Tube rack
• 1 Quick Reference Guide
• 1 Instructions for use data sheet
Materials Required but not Provided
• Personal Protective Equipment per local recommendations (i.e. gown/
lab coat, face mask, face shield/eye goggles and gloves), Timer, Biohazard
container
Active Ingredients of Main Components
• 1 Test device Gold conjugate: Human IgG specific to SARS-CoV-2 Ag gold
colloid and Chicken IgY - gold colloid, Test line: Mouse monoclonal antiSARS-CoV-2, Control line: Mouse monoclonal anti-Chicken IgY
• Buffer Tricine, Sodium Chloride, Tween 20, Sodium Azide (<0.1%), Proclin 300
Storage and Stability
1. The test kit should be stored at a temperature between 2-30 °C. Do not
freeze the kit or its components.
Note: When stored in a refrigerator, all kit components must be brought
to room temperature (15-30 °C) for a minimum of 30 minutes prior to
performing the test. Do not open the pouch while components come to room
temperature.
2. The Buffer bottle may be opened and resealed for each assay. The Buffer cap
should be firmly sealed between each use. The Buffer is stable until expiration
date if kept at 2-30 °C.
3. Perform the test immediately after removing the test device from the foil
pouch.
4. Do not use the test kit beyond its expiration date.
5. The shelf life of the kit is as indicated on the outer package.
6. Do not use the test kit if the pouch is damaged or the seal is broken.
7. Direct swab specimens should be tested immediately after collection. If
immediate testing is not possible, the swab specimen can be kept in an
extraction tube filled with extraction buffer (300 μl) at room temperature
(15-30 °C) for up to two hours prior to testing.
Warnings
1. For in vitro diagnostic use only. Do not reuse the test device and kit
components.
2. These instructions must be strictly followed by a trained healthcare
professional to achieve accurate results. All users have to read the instruction
prior to performing a test.
3. Do not eat or smoke while handling specimens.
4. Wear protective gloves while handling specimens and wash hands thoroughly
afterwards.
5. Avoid splashing or aerosol formation of specimen and buffer.
6. Clean up spills thoroughly using an appropriate disinfectant.
7. Decontaminate and dispose of all specimens, reaction kits and potentially
contaminated materials (i.e. swab, extraction tube, test device) in a biohazard
container as if they were infectious waste and dispose according to applicable
local regulations.
8. Do not mix or interchange different specimens.
9. Do not mix reagent of different lots or those for other products.
10. Do not store the test kit in direct sunlight.
11. To avoid contamination, do not touch the head of provided swab when
opening the swab pouch.
12. The sterilized swabs should be used only for nasal specimen collection.
13. To avoid cross-contamination, do not reuse the sterilized swabs for specimen
collection.
14. Do not dilute the collected swab with any solution except for the provided
extraction buffer.
15. The buffer contains <0.1% sodium azide as a preservative which may be toxic if
ingested. When disposed of through a sink, flush with a large volume of water.7
16. Do not use the positive or negative control swab for specimen collection.
Test Procedure (Refer to Figure)
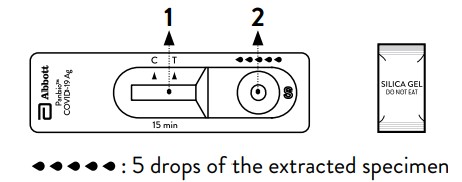
Nasal swab Specimens
Note: Healthcare professionals should comply with personal safety guidelines
including the use of personal protective equipment.
Test Preparation
1. Allow all kit components to reach a temperature between 15-30 °C prior to
testing for 30 minutes.
2. Remove the test device from the foil pouch prior to use. Place on a flat,
horizontal and clean surface.
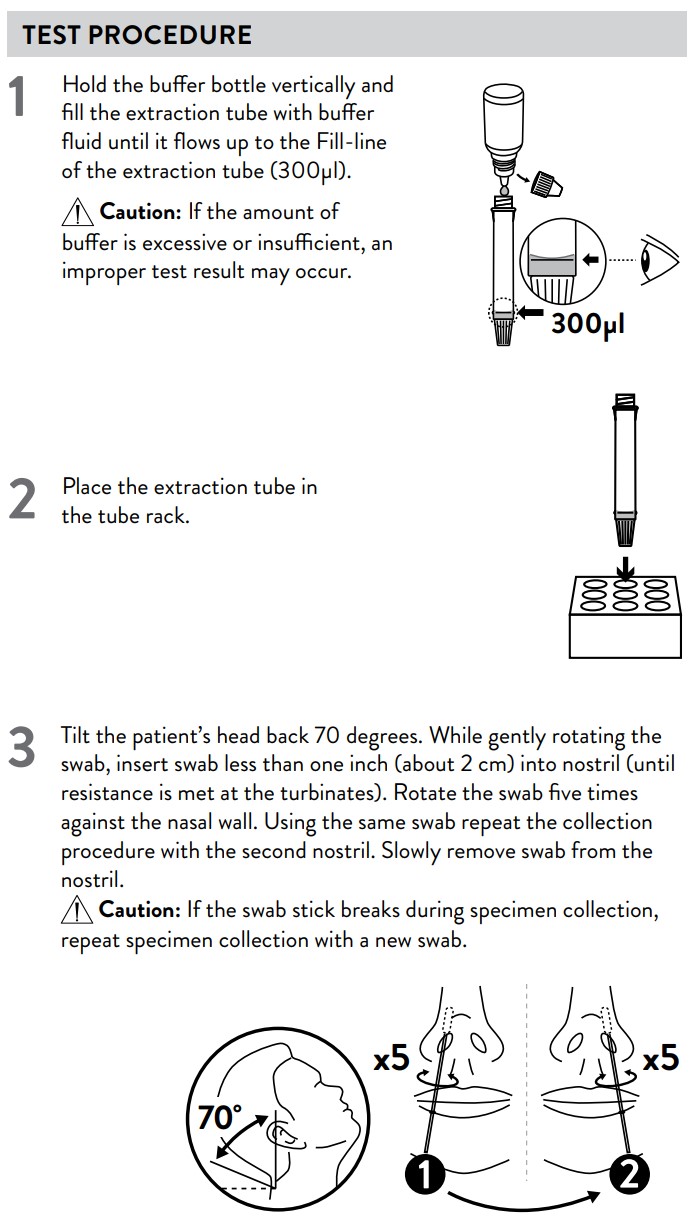
3. Hold the buffer bottle vertically and fill the extraction tube with buffer fluid
until it flows up to the Fill-line of the extraction tube (300 μl).
Caution: If the amount of buffer is excessive or insufficient, an improper
test result may occur.
4. Place the extraction tube in the tube rack.
Nasal Mid-Turbinate (NMT) Specimen Collection & Extraction
1. Tilt the patient’s head back 70 degrees. While gently rotating the swab, insert
swab less than one inch (about 2 cm) into nostril (until resistance is met at
the turbinates).
2. Rotate the swab five times against the nasal wall then slowly remove from
the nostril.
3. Using the same swab repeat the collection procedure with the second nostril.
Caution: If the swab stick breaks during specimen collection, repeat
specimen collection with a new swab.
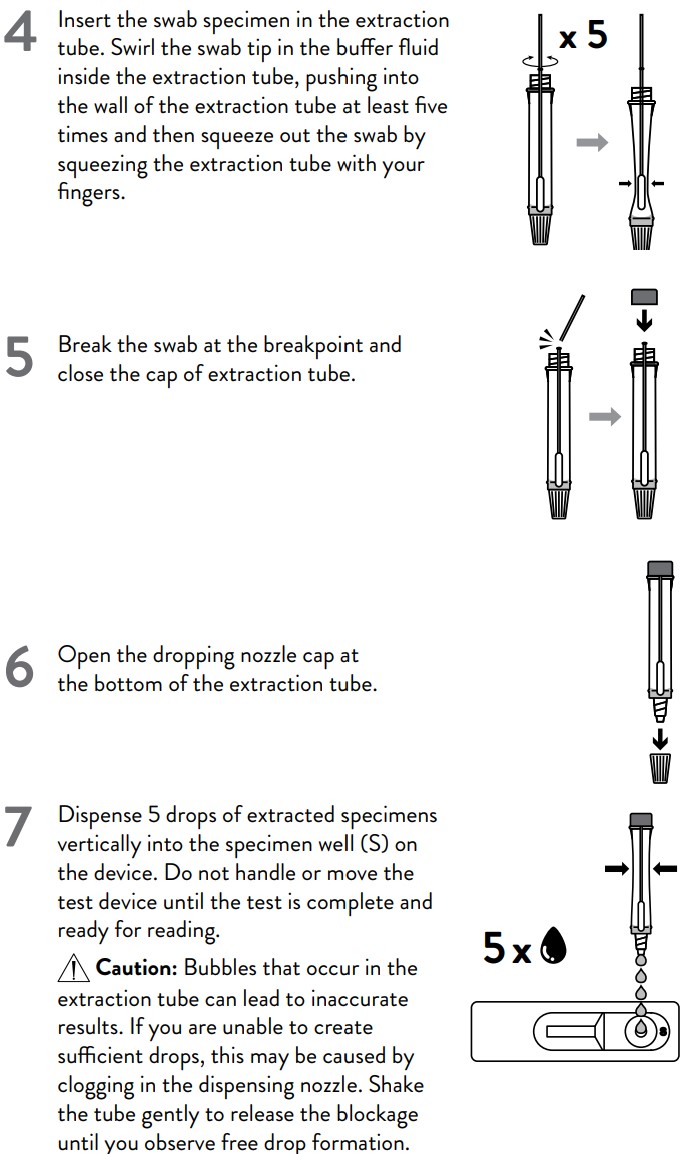
4. Swirl the swab tip in the buffer fluid inside the extraction tube, pushing into
the wall of the extraction tube at least five times and then squeeze out the
swab by squeezing the extraction tube with your fingers.
5. Break the swab at the breakpoint and close the cap of extraction tube.
Reaction with Test Device
1. Open the dropping nozzle cap at the bottom of the extraction tube.
2. Dispense 5 drops of extracted specimens vertically into the specimen well
(S) on the device. Do not handle or move the test device until the test is
complete and ready for reading.
Caution: Bubbles that occur in the extraction tube can lead to inaccurate
results. If you are unable to create sufficient drops, this may be caused by
clogging in the dispensing nozzle. Shake the tube gently to release the
blockage until you observe free drop formation.
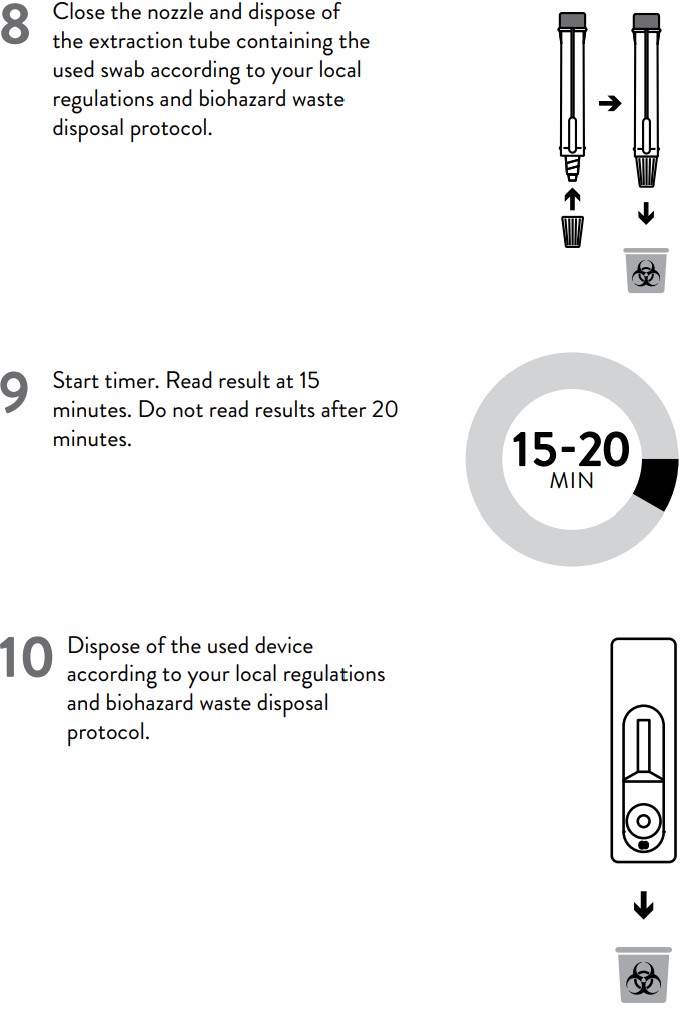
3. Close the nozzle and dispose of the extraction tube containing the used swab
according to your local regulations and biohazard waste disposal protocol.
4. Start timer. Read result at 15 minutes. Do not read results after 20 minutes.
5. Dispose of the used device according to your local regulations and biohazard
waste disposal protocol.
Positive / Negative Control Swab
Caution: Control use only. Do not use the positive or negative control swab for specimen collection.
Note: Please refer to the External Quality Control section of this Instructions for
use for the frequency of testing external quality control swabs.
1. Hold the buffer bottle vertically and fill the extraction tube with buffer fluid
until it flows up to the Fill-line of the extraction tube (300 μl).
Caution: If the amount of buffer is excessive or insufficient, an improper
test result may occur.
2. Place the extraction tube in the tube rack.
3. Insert the positive or negative control swab in the buffer fluid inside of the
extraction tube and soak the swab for 1 minute. Swirl the control swab tip
in the buffer fluid inside of the extraction tube, pushing into the wall of the
extraction tube at least five times and then squeeze out the swab by squeezing
the extraction tube with your fingers.
4. Dispose of the used control swab in accordance with your biohazard waste
disposal protocol.
5. Close the cap of the extraction tube.
6. Follow the above test procedure [Reaction with Test Device].
Test Interpretation (Refer to Figure)
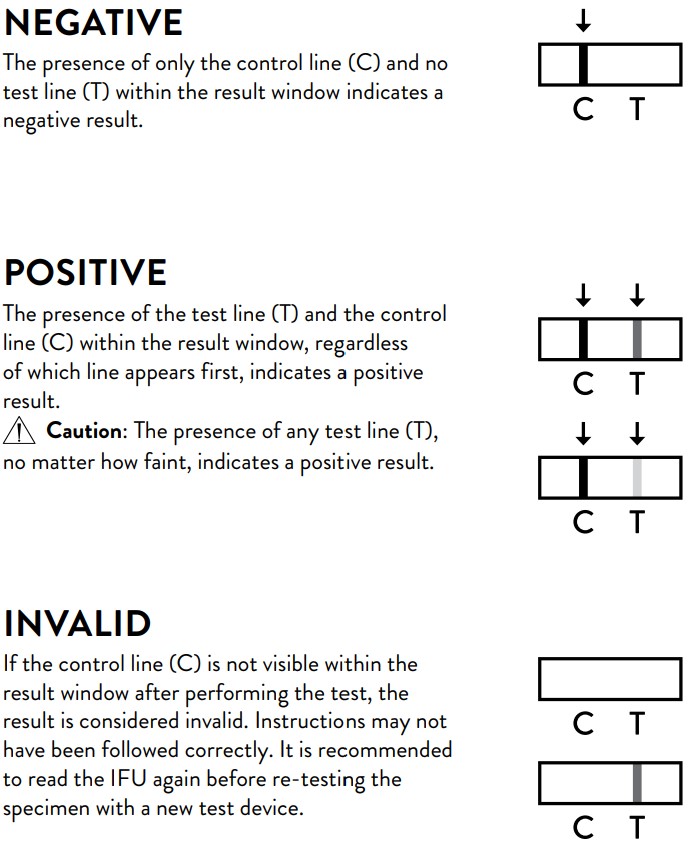
1. Negative result: The presence of only the control line (C) and no test line (T)
within the result window indicates a negative result.
2. Positive result: The presence of the test line (T) and the control line (C)
within the result window, regardless of which line appears first, indicates a
positive result.
Caution: The presence of any test line (T), no matter how faint, indicates
a positive result.
3. Invalid result: If the control line (C) is not visible within the result window
after performing the test, the result is considered invalid.
Test Limitations
1. The contents of this kit are to be used for the professional and qualitative
detection of SARS-CoV-2 antigen from nasal swab. Other specimen types
may lead to incorrect results and must not be used.
2. Failure to follow the instructions for test procedure and interpretation of test
results may adversely affect test performance and/or produce invalid results.
3. A negative test result may occur if the specimen was collected, extracted
or transported improperly. A negative test result does not eliminate the
possibility of SARS-CoV-2 infection and should be confirmed by viral culture
or a molecular assay.
4. Positive test results do not rule out co-infections with other pathogens.
5. Test results must be evaluated in conjunction with other clinical data available
to the physician.
6. Reading the test results earlier than 15 minutes or later than 20 minutes may
give incorrect results.
7. Panbio™ COVID-19 Ag Rapid Test Device is not intended to detect from
defective (non-infectious) virus during the later stages of viral shedding that
might be detected by PCR molecular tests.8
8. Positive results may occur in cases of infection with SARS-CoV.
Quality Control
1. Internal Quality Control:
The test device has a test line (T) and a control line (C) on the surface of the
test device. Neither the test line nor the control line are visible in the result
window before applying a specimen. The control line is used for procedural
control and should always appear if the test procedure is performed properly
and the test reagents of the control line are working.
2. External Quality Control:
The controls are specifically formulated and manufactured to ensure
performance of the Panbio™ COVID-19 Ag Rapid Test Device and are used
to verify the user’s ability to properly perform the test and interpret the results.
The Positive Control contains recombinant SARS-CoV-2 nucleocapsid
protein, which is not contagious. The Positive Control will produce a positive
test result and has been manufactured to produce a visible test line (T). The
Negative Control will produce a negative test result. Control swabs are not
specific for a particular Panbio™ COVID-19 Ag Rapid Test Device lot and
may be used between test device lots until the swabs’ expiry dates.
Good laboratory practice suggests the use of positive and negative controls
to ensure that:
• Test reagents are working, and
• The test is correctly performed.
The external controls can be run under any of the following circumstances:
• By a new operator prior to performing testing on patient specimens,
• When receiving a new test shipment,
• At periodic intervals as dictated by local requirements, and/or by the user’s
Quality Control procedures.
Performance Characteristics
1. External evaluation of Panbio™ COVID-19 Ag Rapid Test Device (Symptomatic)
Clinical performance of Panbio™ COVID-19 Ag Rapid Test Device was
determined by testing 104 positive nasal swab specimens and 404 negative
specimens for SARS-CoV-2 antigen (Ag) to have a sensitivity of 98.1% (95%
CI: 93.2-99.8%) and a specificity of 99.8% (95% CI: 98.6-100.0%). Clinical
specimens were determined to be positive or negative using an FDA EUA
RT-PCR reference method. The individuals on which the reported sensitivity and specificity are based also had a nasopharyngeal swab taken, which was tested in the FDA EUA approved RT-PCR.
Panbio™ COVID-19 Ag Rapid Test Device Results
Nasal PCR Test Result
Positive Negative Total
Panbio™ COVID-19
Ag Rapid Test Device
Result (nasal swab
specimens)
Positive 102 1 103
Negative 2 403 405
Total 104 404 508
Sensitivity Specificity Overall Percent
Agreement
98.1%
[93.2%; 99.8%]
99.8%
[98.6%; 100.0%]
99.4%
[98.3%; 99.9%]
• Performance data was calculated from a study of individuals suspected of
exposure to COVID-19 or who have presented with symptoms in the last
7 days.
• Stratification of the positive specimens post onset of symptoms or suspected
exposure between 0-3 days has a sensitivity of 100.0% (95% CI: 92.3-
100.0%; n=46) and 4-7 days has a sensitivity of 96.6% (95% CI: 88.1-
99.6%; n=58).
• Positive agreement of the Panbio™ COVID-19 Ag Rapid Test Device is
higher with samples of Ct values ≤30 with a sensitivity of 100.0% (95% CI:
96.0-100.0%) and Ct values ≤33 with a sensitivity of 99.0% (95% CI: 94.5-
100.0%). As indicated in References 8-10, patients with Ct value >30 are no
longer contagious. 8, 9, 10
• The clinical performance data was also calculated vs nasopharyngeal swab
specimens using an FDA EUA RT-PCR reference and has a sensitivity
of 91.1% (95% CI: 84.2-95.6%) and specificity of 99.7% (95% CI: 98.6-
100.0%).
2. External evaluation of Panbio™ COVID-19 Ag Rapid Test Device
(Asymptomatic)
Clinical performance of Panbio™ COVID-19 Ag Rapid Test Device was
determined by testing 483 asymptomatic subjects for SARS-CoV-2 antigen
(Ag). Clinical specimens were determined to be positive or negative using an
FDA EUA RT-PCR reference method.
The positive results (n=50) were stratified by the comparator method cycle
threshold (Ct) counts and assessed to better understand the correlation of
product performance, as a surrogate for the amount of virus present in the
clinical sample. A lower Ct value corresponds to a higher virus concentration.
As presented in the table below, the positive agreement increases with lower
Ct values.
The specificity (n=433) was 100% with 95% CI [99.2%; 100.0%].
The results for sensitivity are summarized in the following table:
All Nasal PCR Positive
The following 43 potentially interfering substances have no impact on
Panbio™ COVID-19 Ag Rapid Test Device. The final test concentrations of
the interfering substances are documented in the Table below.
Specimen Interfering Substances Final Test
Concentration Test Result
1
Endogenous
Substance
Mucin 0.5% No Interference
2 Hemoglobin 100 mg/L No Interference
3 Triglycerides 1.5 mg/L No Interference
4 Icteric (Bilirubin) 40 mg/dL No Interference
5 Rheumatoid factor 200 IU/ml No Interference
6 Anti-nuclear antibody >1:40 No Interference
7 Pregnant 10-fold dilution No Interference
8 Exogenous Substance Guaiacol glyceryl ether 1 μg/ml No Interference
9 Albuterol 0.005 mg/dL No Interference
10 Ephedrine 0.1 mg/ml No Interference
11 Chlorpheniramine 0.08 mg/dL No Interference
12 Diphenhydramine 0.08 mg/dL No Interference
13 Ribavirin 26.7 μg /ml No Interference
14 Oseltamivir 0.04 mg/dL No Interference
15 Zanamivir 17.3 μg /ml No Interference
16 Phenylephrine
hydrochloride 15% v/v No Interference
17 Oxymetazolin
hydrochloride 15% v/v No Interference
18 Amoxicillin 5.4 mg/dL No Interference
19 Acetylsalicylic acid 3 mg/dL No Interference
20 Ibuprofen 21.9 mg/dL No Interference
21 Chlorothiazide 2.7 mg/dL No Interference
22 Indapamide 140 ng/ml No Interference
23 Glimepiride
(Sulfonylureas) 0.164 mg/dL No Interference
24 Acarbose 0.03 mg/dL No Interference
25 Ivermectin 4.4 mg/L No Interference
26 Lopinavir 16.4 μg/L No Interference
27 Ritonavir 16.4 μg/L No Interference
28 Chloroquine phosphate 0.99 mg/L No Interference
Exogenous
Substance
Sodium chloride with
preservatives 4.44 mg/ml No Interference
30 Beclomethasone 4.79 ng/ml No Interference
31 Dexamethasone 0.6 µg/ml No Interference
32 Flunisolide 0.61 µg/ml No Interference
33 Triamcinolone 1.18 ng/ml No Interference
34 Budesonide 2.76 ng/ml No Interference
35 Mometasone 1.28 ng/ml No Interference
36 Fluticasone 2.31 ng/ml No Interference
37 Sulfur 9.23 µg/ml No Interference
38 Benzocaine 0.13 mg/ml No Interference
39 Menthol 0.15 mg/ml No Interference
40 Mupirocin 10 µg/ml No Interference
41 Tobramycin 24.03 µg/ml No Interference
42 Biotin 1.2 µg/ml No Interference
43 HAMA 63.0 ng/ml No Interference
8. Repeatability & Reproducibility
Repeatability & Reproducibility of Panbio™ COVID-19 Ag Rapid Test
Device was established using in-house reference panels containing negative
specimens and a range of positive specimens. There were no differences
observed within-run, between-run, between-lots, between-sites, and
between-days.
Allow all kit components to reach a temperature between 15-
30°C prior to testing for 30 minutes.
Note: Healthcare professionals should comply with personal safety
guidelines including the use of personal protective equipment.
Carefully read these instructions prior to using Panbio™
COVID-19 Ag Rapid Test Device kit.
Look at the expiration date of the kit box. If the expiration date
has passed, use another kit.
Open the package and look for the following:
1. Test device with desiccant in individual foil pouch
2. Buffer
3. Extraction tube
4. Extraction tube cap
5. Positive control swab
6. Negative control swab
7. Sterilized nasal swabs for sample collection
8. Tube rack
9. Quick reference guide
10. Instructions for use
TEST PROCEDURE
Insert the swab specimen in the extraction
tube. Swirl the swab tip in the buffer fluid
inside the extraction tube, pushing into
the wall of the extraction tube at least five
times and then squeeze out the swab by
squeezing the extraction tube with your
fingers.
4 x 5
Dispense 5 drops of extracted specimens
vertically into the specimen well (S) on
the device. Do not handle or move the
test device until the test is complete and
ready for reading.
Caution: Bubbles that occur in the
extraction tube can lead to inaccurate
results. If you are unable to create
sufficient drops, this may be caused by
clogging in the dispensing nozzle. Shake
the tube gently to release the blockage
until you observe free drop formation.
TEST INTERPRETATION
The presence of the test line (T) and the control
line (C) within the result window, regardless
of which line appears first, indicates a positive
result.
Caution: The presence of any test line (T),
no matter how faint, indicates a positive result.
If the control line (C) is not visible within the
result window after performing the test, the
result is considered invalid. Instructions may not
have been followed correctly. It is recommended
to read the IFU again before re-testing the
specimen with a new test device.
POSITIVE
INVALID
The presence of only the control line (C) and no
test line (T) within the result window indicates a
negative result.
NEGATIVE
REFERENCES
1. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease
(COVID-19) outbreak. J Autoimmun. 2020; Feb 26:102433. doi: 10.1016/j.jaut.2020.102433.
2. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus
disease 2019 (COVID-19) outbreak-an update on the status. Mil Med Res. 2020; Mar 13;
7(1):11.doi:10.1186/s40779-020-00240-0.
3. Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus 2 (SARSCov-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J
Antimicrob Agents. 2020; Mar 55(3): 105924.doi: 10.1016/j.ijantimicag.2020.105924.
4. In Vitro Diagnostic Assays for COVID-19: Recent Advances and Emerging Trends (Sandeep
Kumar Vashist, 2020 April 05: diagnostics)
5. Nano Research for COVID-19 (http://dx.doi.org/10.1021/acsnano.0c02540)
6. Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid
Detection of the Virus that Causes COVID-19 in Patients (Stephen M, Hahn M.D. 2020 May
09: Commisioner of Food and Drugs
7. Current Intelligence Bulletin 13: Explosive Azide Hazard DHHS (NIOSH) Publication Number
78-127 August 16, 1976
8. CDC. Discontinuation of Transmission-Based Precautions and Disposition of Patients with
COVID-19 in Healthcare Settings (Interim Guidance). (2020).
9. CDC. Duration of Isolation and Precautions for Adults with COVID-19. (2020).
10. Bullard, et al. Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From
Diagnostic Samples. CID. 2020; Nov 15;71 (10); DOI:10.1093/cid/ciaa638
Temperature limitation
For in vitro diagnostic use only
Do not reuse
Do not use if package is damaged
GLOSSARY OF SYMBOLS
- Lot Number
- Catalog Number
- Consult instructions for use
- Keep dry
- Biological Risks
- Use By
- Manufacturer
- Date of manufacture
- Keep away from sunlight
- CE mark
- Contains sufficient for X tests
- Sterilized using ethylene oxide
- Sterilized using irradiation
- Do not re-sterilize
- Negative control
- Positive control
Videos Hide Videos Show Videos
-

Panbio Nasal Swab
Panbio Nasal 25 tests Abbott 41fk11 https://gentaur.co.uk/shop...
-

Panbio Test Procedure
Home test for Antigen with Abbott https://www.genprice.eu/panb...
-

Panbio Antigen Nasal Swab Procedure
41FK11 is the easiest test for children. The swab goes less de...
-

self swab how to do?
Saliva swabs are in the mouth Nasal swabs are in the nose Naso...
-

Panbio Home Nasopharyngeal Swab Procedure for deep nose test
41FK10 NasoPharingeal needs to be put deep into the Nose It is...





























